It’s 06:30 when EMS is called to an inner-city apartment for an 18-year-old male having a seizure. After gaining entry into the building, Paramedics and First Responders trudge up two flights of stairs and down a narrow, dimly lit hallway, until they find an open door into a dark two-bedroom apartment. There are three people in the living room, all about 18-years-old, and one of them is lying awkwardly on the floor, propped up against the side of the couch. There is evidence of extensive alcohol and drug consumption littering the scene.
It proves difficult to obtain a complete patient history, since bystanders on scene are still somewhat inebriated, and they remain apprehensive about cooperating with emergency responders. However, the story gathered is as follows.
The three of them were up all night partying, drinking, and ingesting multiple illicit substances. It is reported that the patient was witnessed to have ingested copious amounts of cocaine, “DXM” (Dextromethorphan, or ‘cough syrup’), marijuana, nicotine, and alcohol. Precise amounts are vague, and it’s possible that there may have been even more ingestions that were not reported. The party ended, and they all went to sleep, until one of the party-goers found the patient seizing on the floor at about 06:30 this morning. It’s unknown how long the patient was down prior to being found.
The patient’s medical history is unknown, though it is believed that he is generally a “pretty healthy guy”.
The patient is unresponsive to any stimuli, has a weak and agonal respiratory effort, and a faint and slow carotid pulse. His general appearance is poor, with significant pallor and central cyanosis noted, though he is hot to the touch.
Crews began administering high-quality 2-person BVM ventilation, utilizing a jaw thrust while inserting an OPA, and positioning the patient with padding behind the head to support a proper ear-to-sternal-notch alignment. Intravenous access, fluid resuscitation, reoxygenation, and basic cardiac monitoring is being maintained while extrication from the apartment is coordinated.
His initial vital signs are as follows:
HR – 60/min, and increasing to 100/min once oxygenation and ventilation is administered
RR – 6/minute and ineffective
SpO2 – Initially <50%, improving to 83% with oxygenation and ventilation
NIBP – 66/34 [44]
Pupils – 6mm bilaterally, extremely sluggish
BGL – 5.0 mmol/L (90 mg/dl)
Temp – 38.5 Celsius (101.3 F)
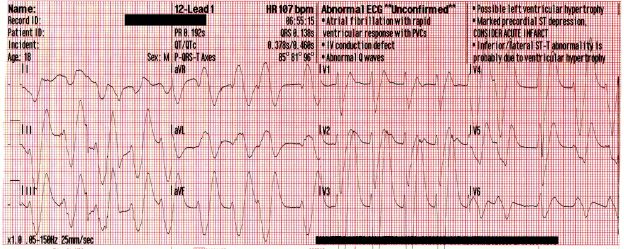
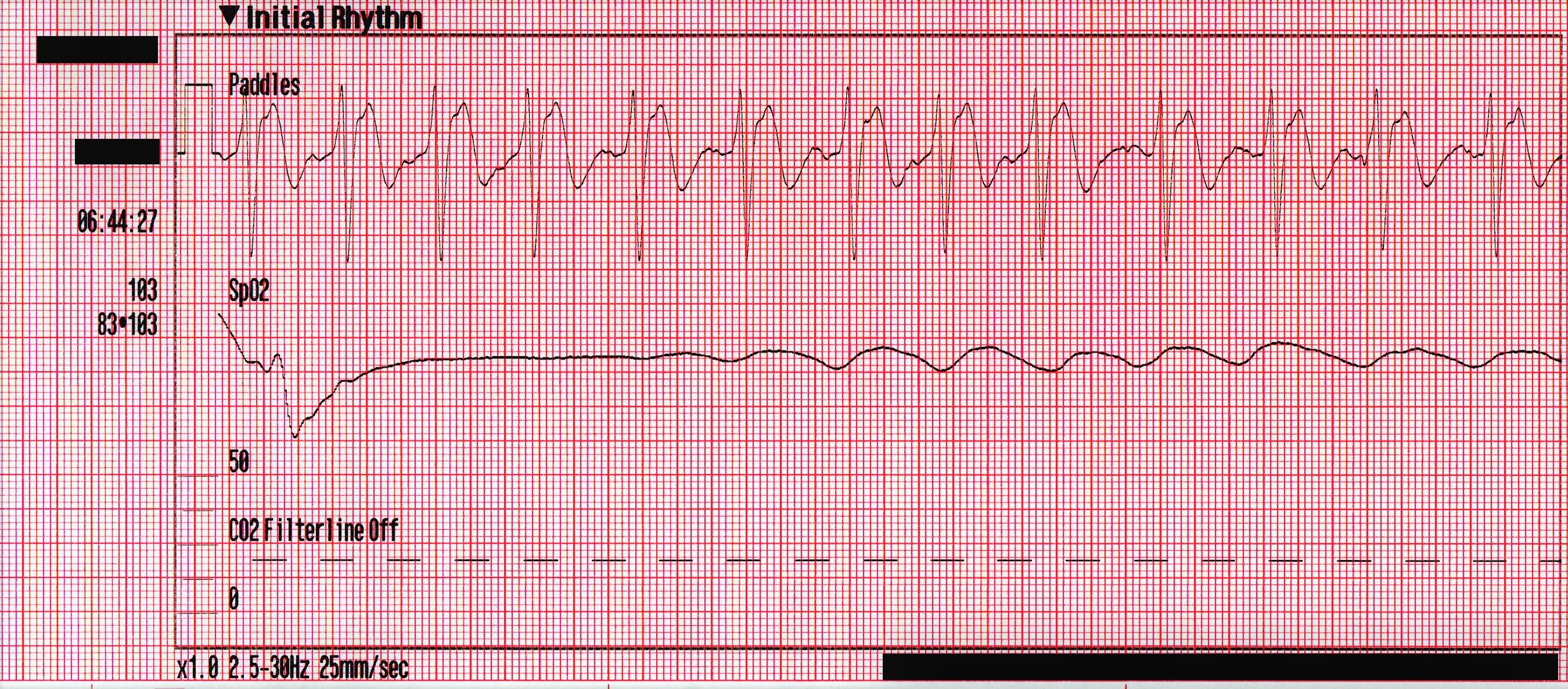
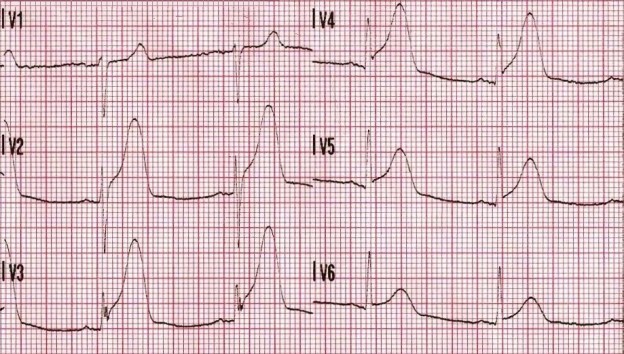
The following rhythm is observed on the monitor. Shortly later, the patient has a generalized seizure which lasts approximately 5 minutes.
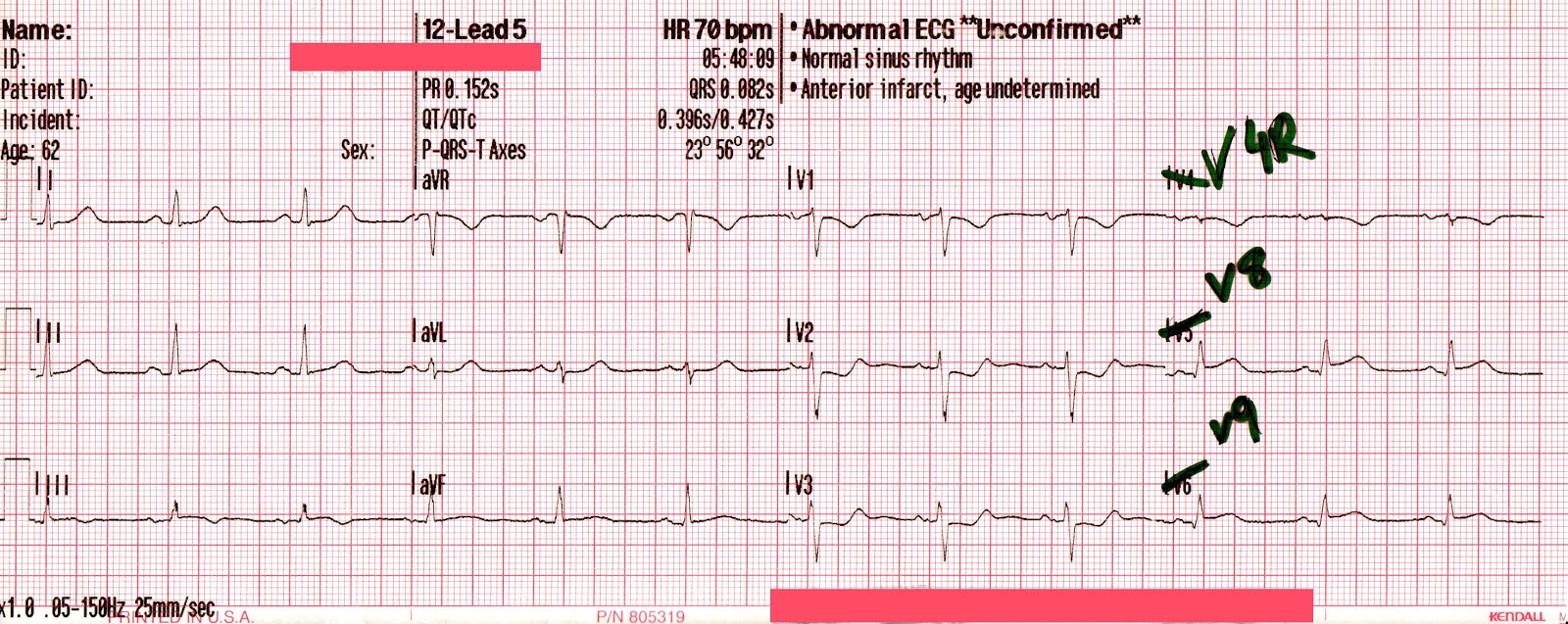
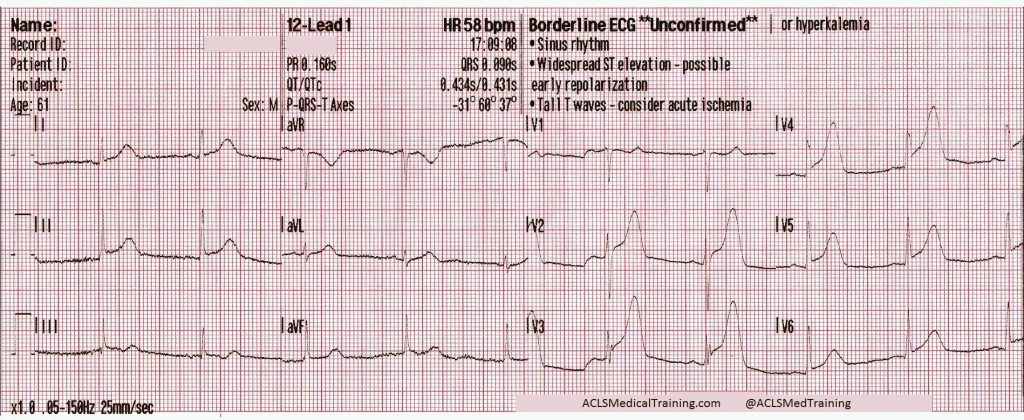
Due to access limitations, the stretcher could not be brought inside to the patient, and so the team of paramedics, police officers and firefighters worked together to move the patient to the ambulance via a device not unlike a large tarp with handles. Once in the ambulance, passive cooling is initiated, and a supraglottic airway is placed. His blood pressure has improved to 87/44 [59], his SpO2 remains in the mid-80’s, and his initial ETCO2 is 86mmHg. The following 12-lead is acquired:
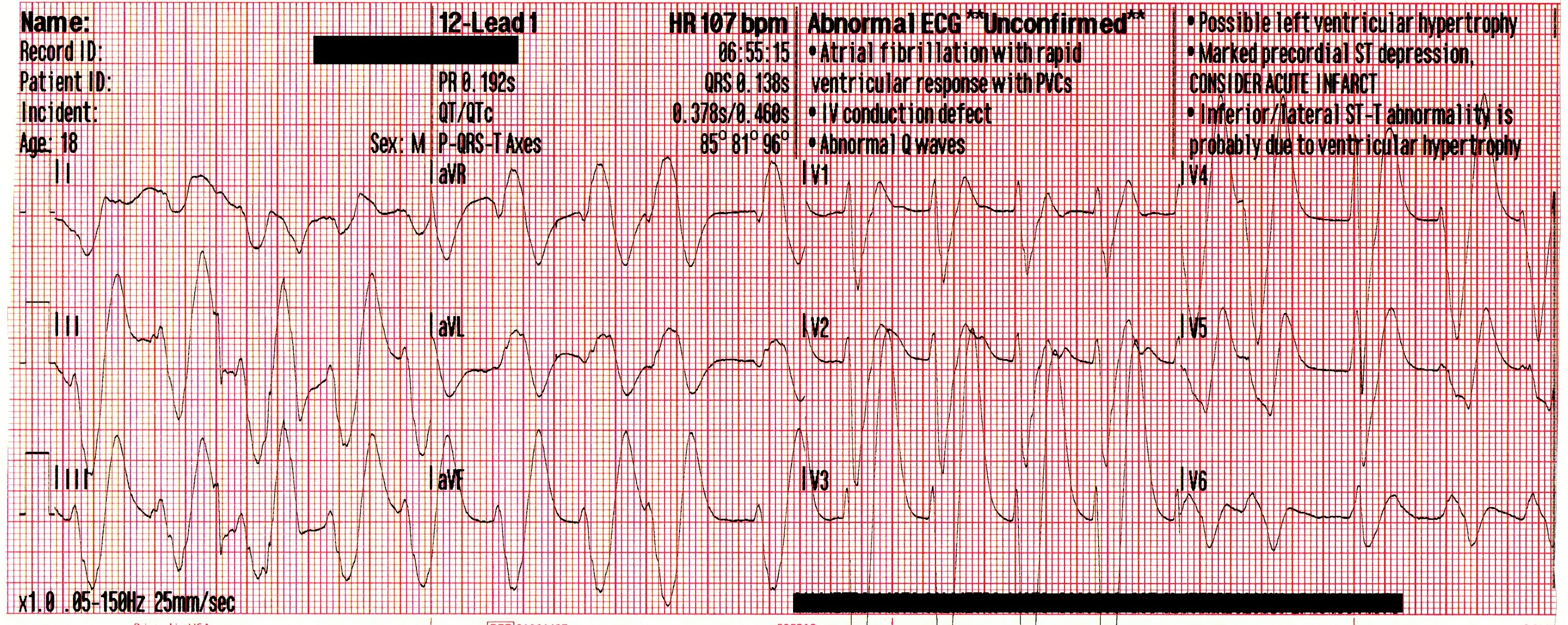
A significant wide-complex tachycardia that is irregularly irregular, with an extreme right axis deviation and a massive terminal R-wave in aVR measuring 10mm. Given the patient’s suspected ingestions and current clinical condition, this ECG should be considered pathognomonic for severe sodium-channel blockade being complicated by extreme acidosis.
Paramedics identified this arrhythmia to most-likely be a complication of the cocaine toxicity, and treatment was aimed at hyperventilation and administration of intravenous sodium bicarbonate (NaHCO3) to correct the acidosis.
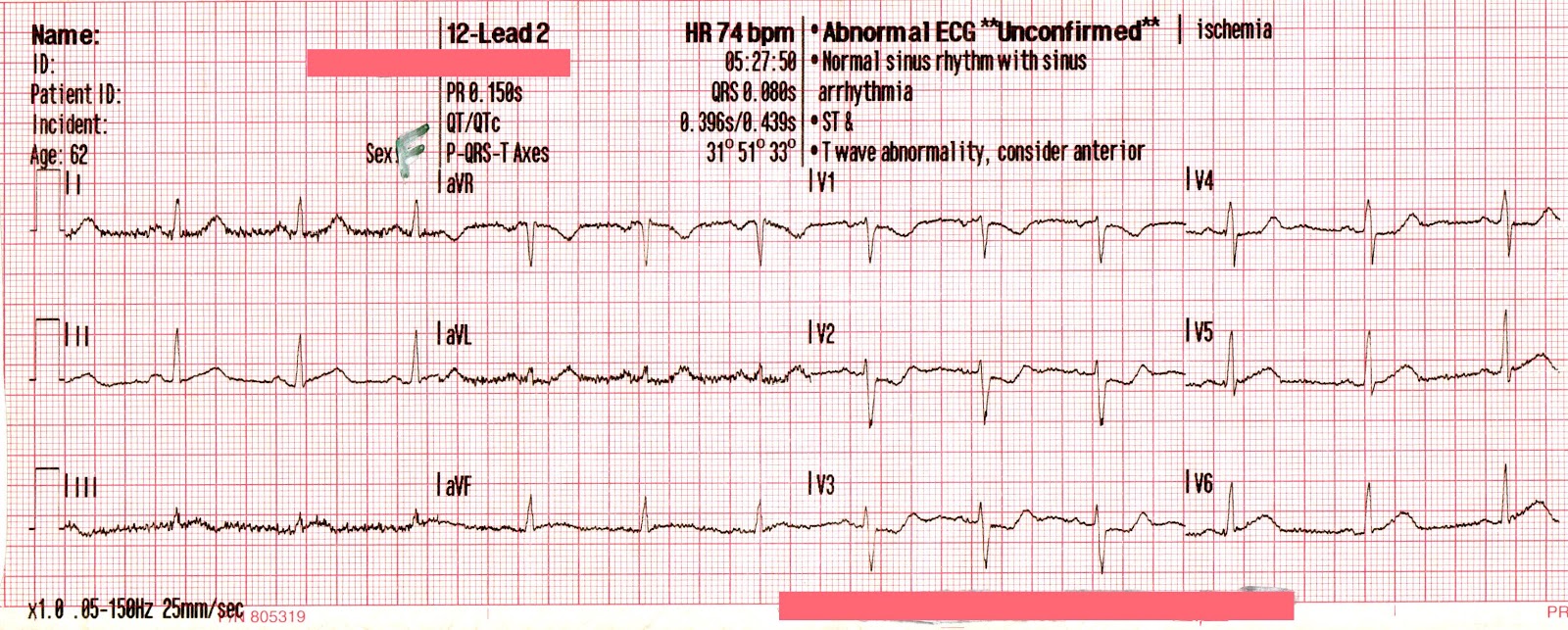
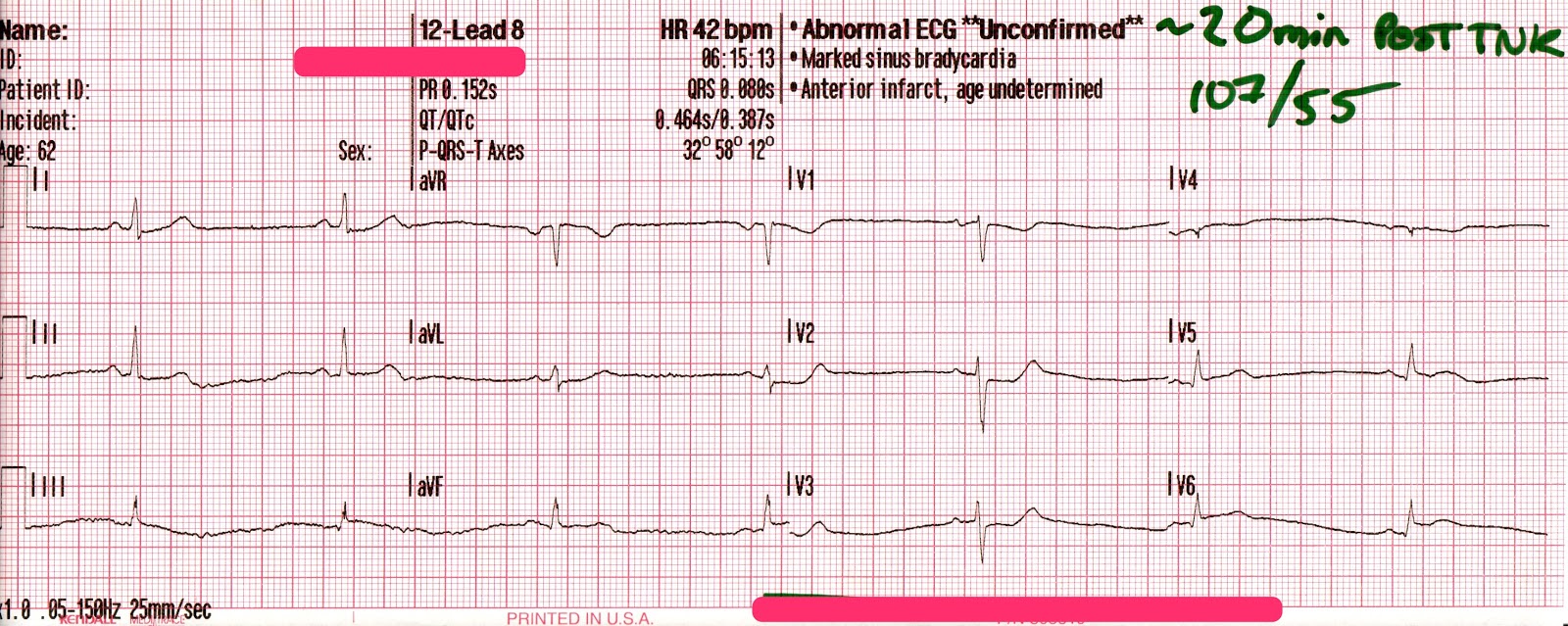
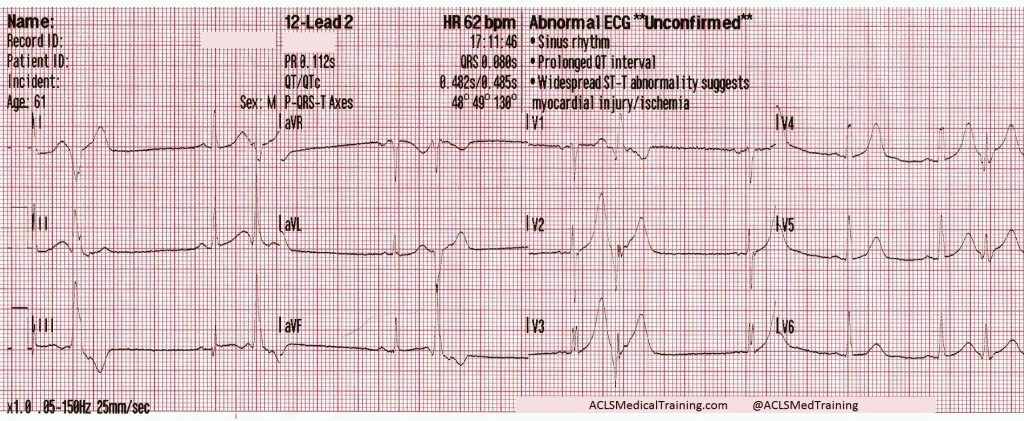
The following 12-lead was recorded following the administration of one amp of 50mEq of NaHCO3 with ongoing attempts at hyperventilation.
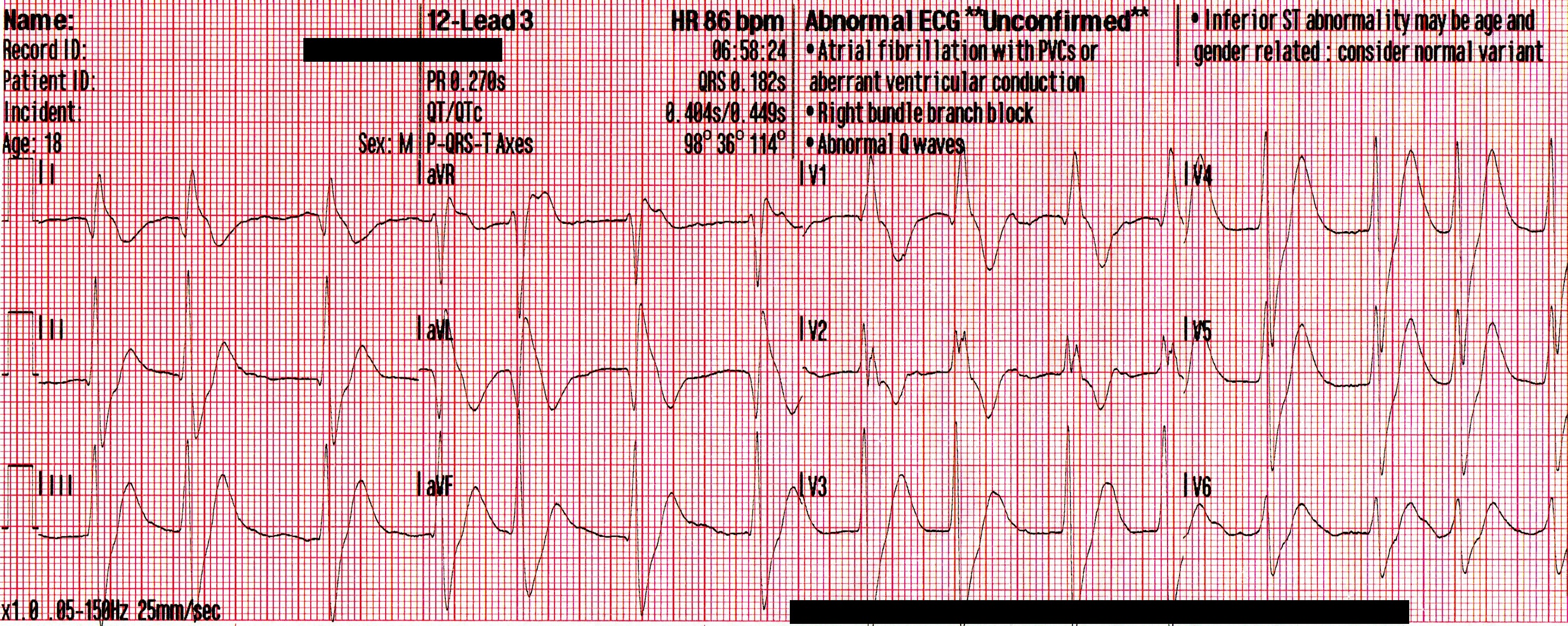
An irregularly-irregular wide-complex rhythm, with an apparent RBBB pattern and peaked T-waves reminiscent of hyperkalemia. This is an improvement, but there are still signs of significant sodium channel blockade.
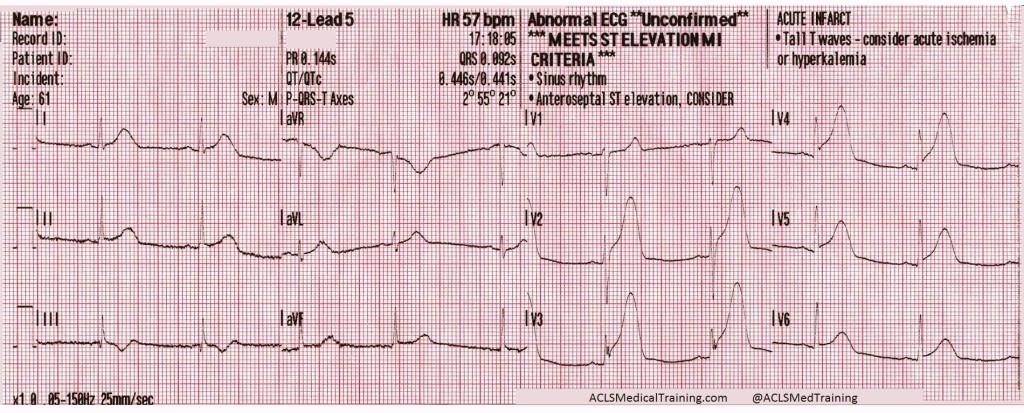
The patient’s SpO2 improved to 100%, his blood pressure remained 85/35 [55], and despite being ventilated at a rate of 30/minute his ETCO2 remained 86mmHg. Another 50mEq of NaHCO3 is administered, and the following 12-lead is acquired:
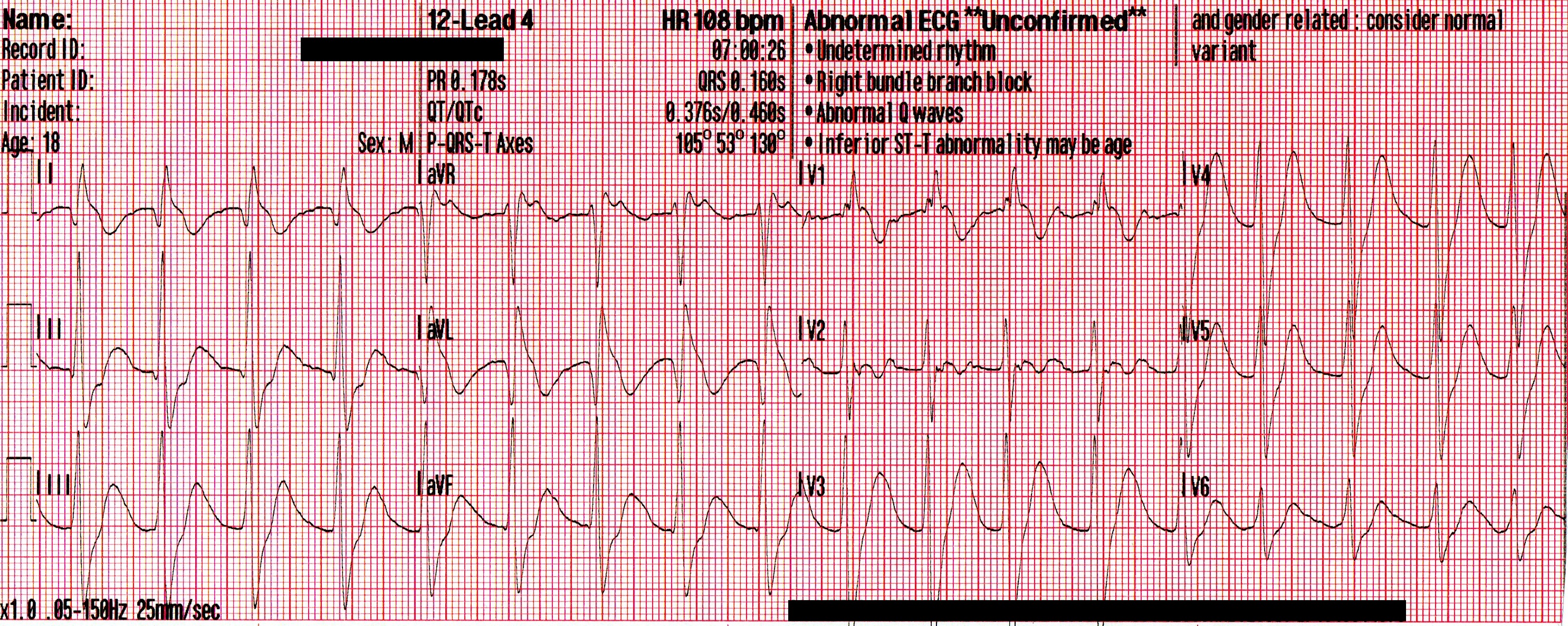
A regular, wide-complex rhythm, with a similar QRS morphology to the previous 12-lead. The QRS is gradually narrowing, but remains pathological.
Conclusion
The crew arrived at the hospital shortly after the second amp of NaHCO3 was given. The ED staff continued administering subsequent doses of NaHCO3 , a peripheral vasopressor (norepinephrine) was initiated, and he was intubated and placed on a ventilator. Initial arterial blood gases revealed a pH of <6.8, pCO2 of >100mmHg, and a lactate of >20mmol/L. He was sent for a CT-head, which revealed no obvious findings of hemorrhage or anoxic brain injury.
He was admitted to ICU, and his repeat ABG thirty minutes later revealed an improved pH of 7.28 and pCO2 of 48mmHg. Unfortunately, no further follow-up was made available to the author.
Discussion
The critically ill toxicology patient can present many unique challenges to prehospital and ED professionals alike. Obstacles often present themselves simultaneously, including airway compromise, cardiac dysrhythmias, and hemodynamic collapse. In an unconscious patient, this is further complicated by unknown co-ingestions, quantities, and comorbidities.
This patient’s presentation can likely be explained by the complex interaction between each of the substances that were ingested. Cocaine mixed with alcohol forms cocaethylene when metabolized by the liver; a substance that’s significantly more cardiotoxic, and possesses a half-life 3-5 times that of cocaine alone. Amongst it’s multiple mechanisms, it acts as a Class Ic sodium-channel blocker, which is represented on the ECG as a progressive widening of the QRS complexes, and the development of an extreme rightward axis in the frontal plane. These channel-toxic effects are amplified by increases in heart rate and decreases in pH – two elements that are found in spades for this young man.
The deleterious effects of the cocaethylene, combined with the ingestion of significant amounts of dextromethorphan; an antitussive and a NMDA-receptor antagonist; would likely result in euphoria, tachycardia, hypertension, dissociation, a decreasing level of consciousness, and potentially severe serotonin syndrome. Hyperthermia, tachycardia, and a disrupted respiratory drive would lead to hypercapnia, worsening acidosis, and a decreased seizure threshold. Left unchecked, this would predictably spiral into a self-perpetuating loop, inevitably resulting in profound shock and hemodynamic collapse.
Treating a patient like this with intravenous sodium bicarbonate (NaHCO3) provides a multi-pronged attack. Following administration, there’s an rapid dissociation of NaHCO3 into Na + HCO3. The extra sodium acts to “overload” the sodium-channels blocked, while the bicarbonate acts as a buffer and binds with free hydrogen (H+) ions to form Carbonic Acid (H2CO3), which then dissociates into water and carbon dioxide, expressed as HCO3 + H → H2CO3 → H2O + CO2. This allows for respiratory correction of the acidosis, and the subsequent alkalinization of the blood helps to reduce the channel-toxic effects of the cocaine. It should be noted, however, that this requires an increased rate of ventilation to ensure adequate elimination of the rising CO2 levels that will follow.
In a case as advanced as this one, where severe decompensated shock has developed, stabilization becomes a delicate and complex hurdle. Since our initial treatments are aimed at alkalinization of the blood to reduce cardiotoxicity, there is a resultant left-shift of the oxyhemoglobin dissociation curve, and that leads to a decreased ability for oxygen to offload from the hemoglobin at the level of the tissue beds. This could potentially hamper our attempts to correct the massive hypoxia that’s developed, and so management is usually targeted at a pH of no higher than 7.50-7.55.
Intubation of this patient would also prove delicate, since critical hypotension and acidosis would likely be worsened by the use of most induction agents or paralytics, forcing providers to classify this as a physiologically difficult airway. For this reason, airway management should likely be accomplished using a resuscitate-before-you-intubate approach. Fluid resuscitation should be well underway before RSI, push-dose pressors should be at the ready, and providers should be aware that there’s a high-likelihood of this patient requiring vasopressor support, despite receiving a 20ml/kg crystalloid bolus.
In conclusion, the critically ill mixed-overdose patient requires aggressive yet calculated emergency management from first responders and physicians alike. A clinical understanding of the pathophysiology, as well as the implications of each aspect of treatment, is vitally important in caring for each of these patients.
Further reading on the subject
Cocaine Overdose Presents with Wide Complex Tachycardia – Alec Weir, M.D. ACLSMedicalTraining.com/Blog (2016)
Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias – Michael E. O’Leary & Jules C. Hancox. British Journal of Clinical Pharmacology (Oct 2009)
Current Concepts: The Serotonin Syndrome – Edward W. Boyer M.D., Ph.D., Michael Shannon M.D., M.P.H. NEJM (2005)
Treatment of patients with cocaine-induced arrhythmias: bringing the bench to the bedside – Robert S Hoffman Br J Clin Pharmacol. (2010)
Tricyclic Overdose (Sodium-Channel Blocker Toxicity) – Edward Burns, M.D. LifeInTheFastLane.com