Differentiating between SVT with aberrancy and ventricular tachycardia (VT) can be a challenging task, even for experienced healthcare providers. This blog aims to clarify the distinction between these two cardiac arrhythmias, emphasizing the potential dangers of misdiagnosis and the importance of accurate identification. By understanding the concepts of aberrant conduction and the characteristics of SVT and VT, healthcare professionals can improve patient care and outcomes.
What Is SVT With Aberrancy?
The term “SVT with aberrancy” tends to throw many providers off, so let’s start by defining SVT using the 2015 ACC/AHA/HRS Guidelines as a reference.
“An umbrella term used to describe tachycardias (atrial and/or ventricular rates in excess of 100 bpm at rest), the mechanism of which involves tissue from the His bundle or above. These SVTs include inappropriate sinus tachycardia, AT (including focal and multifocal AT), macroreentrant AT (including typical atrial flutter), junctional tachycardia, AVNRT, and various forms of accessory pathway-mediated reentrant tachycardias. In this guideline, the term does not include AF.”
This is important because many of us were taught a narrow, complex rhythm: “must be SVT if the rate is over 150,” which can lead to inappropriate therapies. In reality, sinus tachycardia is a form of SVT, and the rate can easily exceed 150. A good rule of thumb to estimate the maximum sinus rate is 220 minus age, but that can vary by 10-15%, which is a lot.
What most people really mean when they call a rhythm “SVT” is AV Nodal Reentrant Tachycardia or AVNRT, which is a reentrant rhythm in or around the AV node. This arrhythmia is usually stable, and the prognosis is much more favorable than that of VT. It is usually treated with vagal maneuvers or adenosine.
What Does Aberrancy Mean?
You can think of “aberrancy” as abnormal conduction. When something is aberrant, it “departs from the right, normal, or usual course.”
Because the right bundle branch tends to have a slightly longer refractory period than the left bundle branch, at higher rates the right bundle branch may not be fully recovered from the previous cardiac cycle, which results in a right bundle branch block pattern.
Even though right bundle branch block aberrancy is more common than left bundle branch block aberrancy, both are possible. Additionally, we know that many patients have underlying bundle branch block, including bifascicular block, at baseline.
When a patient with a bundle branch block experiences SVT, the result is a wide complex tachycardia.
Is Aberrant Conduction Dangerous?
While aberrant conduction itself is not inherently dangerous, the potential for misdiagnosis is a significant concern. Mistaking SVT with aberrancy for VT can lead to the administration of antiarrhythmic medications or cardioversion, which can be harmful if the underlying rhythm is SVT.
SVT With Aberrancy vs. VT
Differentiating between SVT with aberrancy and VT can help determine the appropriate treatment. Several factors can help distinguish between the two, including the patient’s clinical presentation, the appearance of the ECG, and the response to specific medications. While there are diagnostic criteria to help rule in VT, there are currently no definitive criteria to safely rule out VT.
It is important to approach wide complex tachycardias with caution and to consider the possibility of both SVT with aberrancy and VT. Obtaining a 12-lead ECG before and after treatment can aid in diagnosis. In cases of unstable wide complex tachycardia, immediate synchronized cardioversion is often necessary.
Can You Differentiate Between SVT With Aberrant Conduction and VT?
The short answer is yes, but it can be very difficult, and even experienced clinicians can misdiagnose VT as SVT with aberrancy!
This can lead to clinical misadventure. In particular, treating a wide complex tachycardia with a calcium channel blocker is a dangerous decision that could have fatal consequences for your patient.
There are good criteria to help rule-in, or tip the scales in favor of VT, but none to safely rule-out VT.
See also: Myths and Cognitive Biases in Interpretation of Wide Complex Tachycardias
Consider the following case:
EMS is dispatched to an 83-year-old female who contacts 9-1-1 after she wakes up with a “racing heart” and shortness of breath.
Past medical history includes myocardial infarction and hypertension.
On initial assessment the patient is found to be alert and oriented to person, place, time, and event. The skin is pale but warm and dry. Radial pulses are very rapid but surprisingly strong. Breath sounds are clear bilaterally.
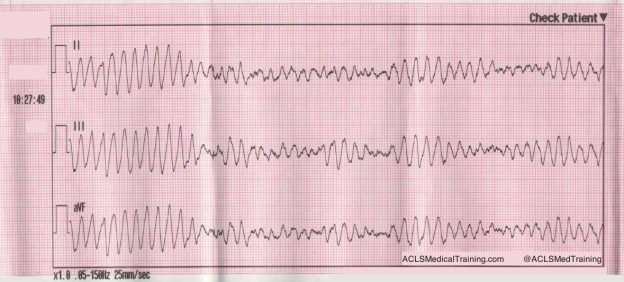
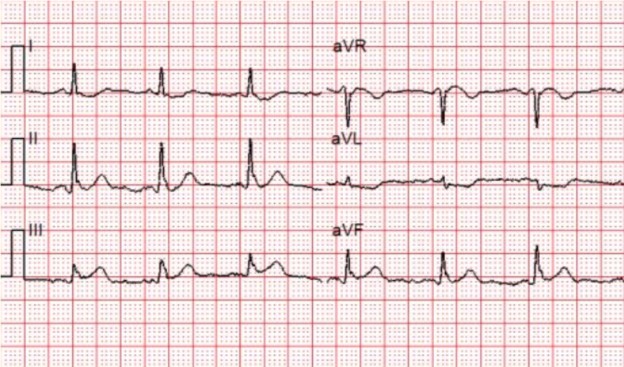
She is placed on the cardiac monitor, and the following rhythm strip is obtained.
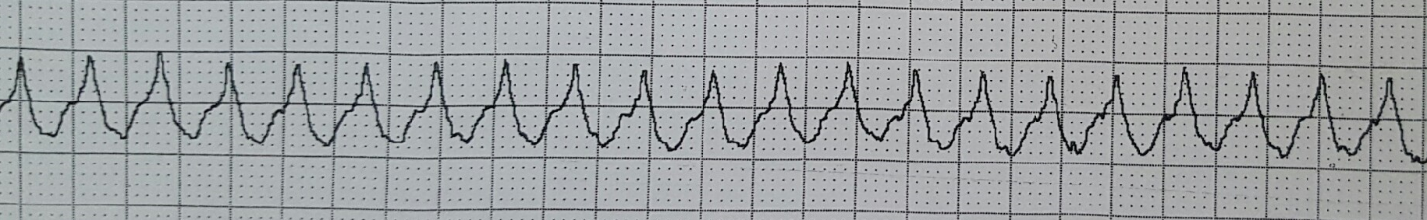
Figure 1: There is a wide and regular complex tachycardia at a rate of ~ 230 bpm.
The patient is placed on oxygen via nasal cannula, and IV access is established while vital signs are obtained.
- RR: 24
- HR: Too fast to count
- NIBP: 112/72
- SpO2: 97%
- Temp: 98.3 F / 36.8 C
Why should you presume that this rhythm is ventricular tachycardia?
- VT accounts for 80% of all cases of WCT
- If the patient has a previous cardiac history, the predictive value can go up over 90%
- An age greater than 35 years has a sensitivity of 92%
Treatment
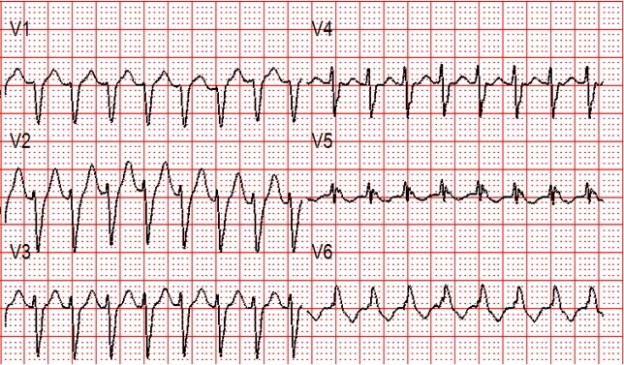
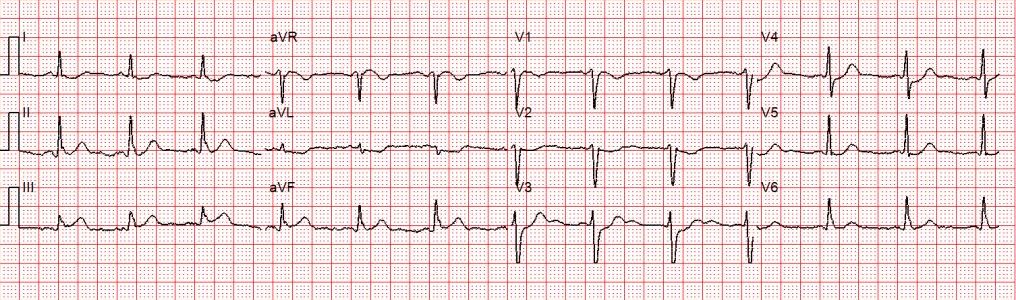
A 12 lead ECG is obtained.
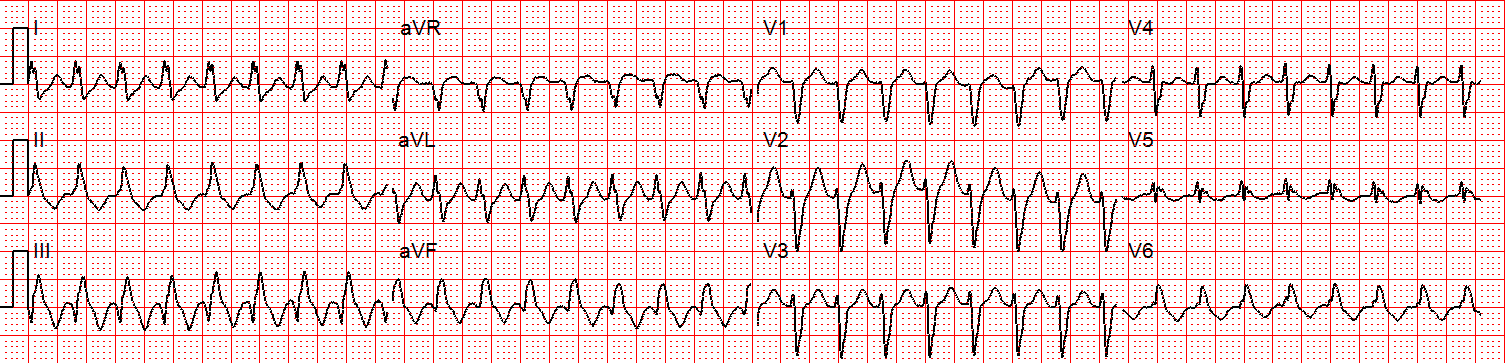
Figure 2: There is a regular wide complex tachycardia at a rate of about 230 without sinus P waves. There is a LBBB pattern in lead V1. However, we would not consider this to be a “typical” LBBB pattern due to the normal axis in the frontal plane and the presence of a small S-wave in lead I.
Amiodarone 150 mg is given over 10 minutes.
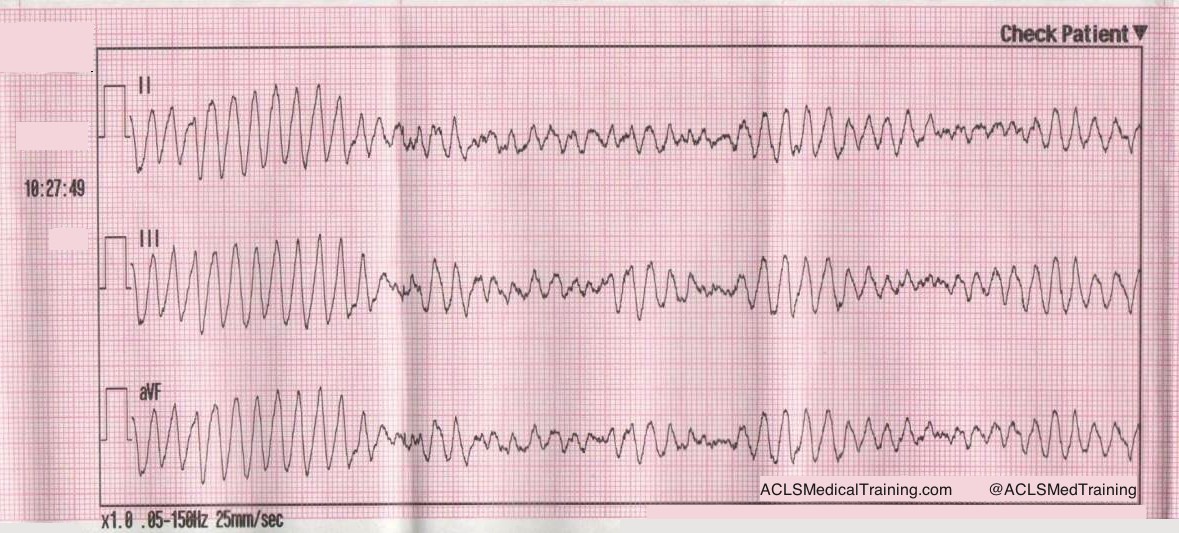
A rhythm change is noted, and the following 12-lead ECG is obtained.
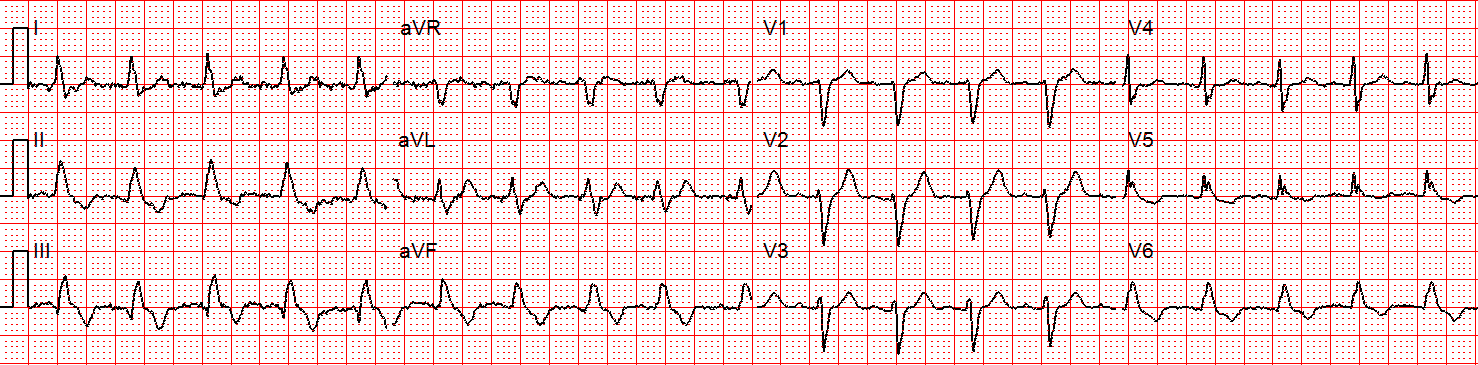
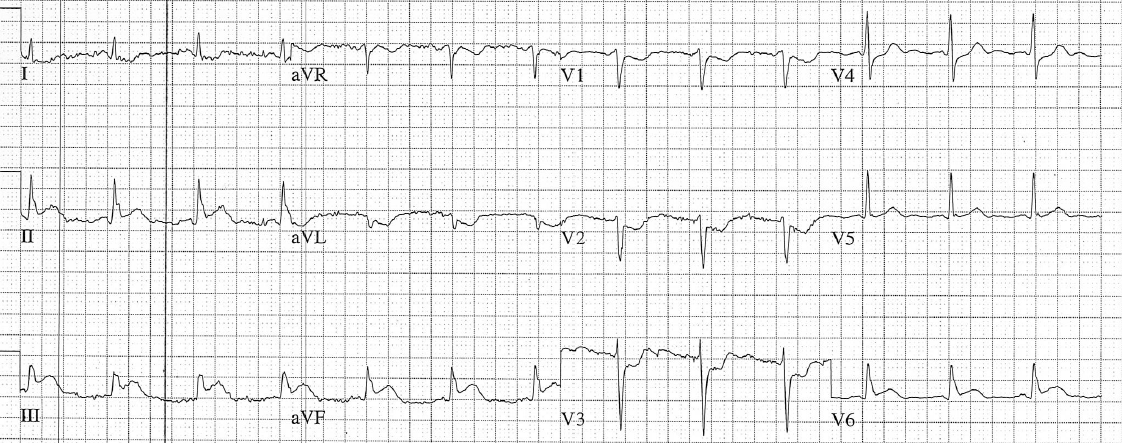
Figure 3: Now there is sinus tachycardia with virtually identical QRS morphology.
Once the patient converts to sinus tachycardia (and after a sigh of relief), paramedics compare the two 12-lead ECGs. The axis and QRS morphology are noted to be exactly the same.
The diagnosis? SVT with aberrancy!
It is safe to conclude that this patient had a conduction defect at baseline, which is what caused the complexes to be wide during the tachycardia.
Retrospectively, adenosine would have been safe and likely effective. In many cases, it can be considered as a first line therapy for undifferentiated wide complex tachycardia, and may have some diagnostic utility when considered in the context of other findings.
Take the Next Step in Cardiac Care With ACLS Medical Training
Accurately differentiating between SVT with aberrancy and ventricular tachycardia is crucial for effective and safe patient care. Misdiagnosis can have severe consequences. By understanding the key characteristics of each arrhythmia and utilizing appropriate diagnostic tools, healthcare providers can improve their ability to recognize and manage these complex cardiac conditions.
To further enhance your knowledge and skills in advanced cardiac life support, consider enrolling in our online ACLS certification course. By investing in your ACLS certification, you’ll be better equipped to handle a wide range of cardiac emergencies and provide optimal care for your patients. Head to our website to get started today!
References
Alzand BCrijns H. Diagnostic criteria of broad QRS complex tachycardia: decades of evolution. Europace. 2010;13(4):465-472
Neumar R, Otto C, Link M et al. Part 8: Adult Advanced Cardiovascular Life Support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18_suppl_3):S729-S767