When trying to decide on a subject for this blog post, I remembered an article I read a few months ago originally published in the Journal of Paramedic Practice, by Logarajah and Alinier titled ‘An Integrated ABCDE Approach To Managing Medical Emergencies Using CRM Principles’.
At the time I first read this article I was lucky enough to have the experience of undertaking a secondment on a Critical Care Paramedic unit and had attended a number of high acuity incidents, and felt the principles outlined assisted me in preparing when heading to these.
I feel using a structured approach can help break down the mental workload, especially when applied to incidents that involve a large degree of decision-making and prioritization. The principles described can be applied to all incidents you may attend as a Paramedic, however in keeping with the increasing focus on human factors and CRM in cardiac arrest management I have decided to tailor them to this area.
Its 17:00pm and nearing the end point of a tiring weekend day shift, just after arriving back on station for the first time since you left in the early hours of the morning you are called to a 51 year old male reported to be in cardiac arrest. Two local community responders and a Technician crew are also attending however you are the only Paramedic available. As you drive to the scene you start to mentally plan how you will approach this incident, what will you need to do, where will you transport the patient to…
ABCDE (alongside remembering how your crewmate likes their coffee) is a cornerstone of all levels of emergency medical care and the tried, tested and trusted tool we all use when we don’t know what to do next. Logarajah and Alinier (2014) proposed a modified version of this mnemonic, using CRM principles, as a tool ‘to remember the sequence in which to manage emergencies or difficult situations while ensuring effective and safe teamwork’ (pp.625).
These principles, in relation to thinking about cardiac arrest management, are as follows:
A: AWARENESS, ANTICIPATION & ALLOCATION OF ATTENTION
What details do you have? What are you anticipating you will find?
Awareness can be divided into two areas; self-awareness and situational awareness. Consideration of both these is vital to maintain control of complex situations and prevent the unanticipated development of additional problems. Anticipating what you will encounter on arrival at the scene is highly dependent on how much or little information you have received.
Try and think about what you would expect to find based upon factors such as the type of incident, the location, or the likely cause of cardiac arrest. This can help prepare you with making decisions such as what equipment to take in with you, treatment algorithms to use and logistical issues such as the locations of nearby hospitals and cath labs.
If you have received an update from resources already on scene you can start to plan how you are going to allocate your clinical attention once you arrive and discuss your plan of action.
B: ‘BE IN THE REQUIRED ROLE’ & BEHAVIOUR
What do you need to do for the team? How can you make the most of your skills? Are you required to be a leader or a follower?
Some of this is dependent on your clinical role however everybody needs to build and maintain a team effort. Does your service use a ‘pit-crew’ CPR approach? Knowing the role you need to assume beforehand can save vital time in decision-making and reduce disruption to already established and organized resuscitation attempts.
C: CALL FOR HELP, COMMUNICATION & COGNITIVE AIDS/CHECKLISTS
Are you going to be able to communicate effectively? What barriers will there be?
Factors such as noise, incidents involving multiple patients, a chaotic scene and poor lighting can all contribute to poor communication. Check your radio, do you know the correct channel to pass updates on? Use your awareness and anticipation to request extra assistance, such as enhanced care teams, early if you think it may be needed.
‘Medicine is not a memory game’
Ensure you have cognitive aids available. Cardiac arrest checklists, resuscitation algorithms and clinical guidelines all can save bandwidth and enable you and the team to focus on the task at hand without having to perform complex mental calculations such as drug doses or tube lengths.
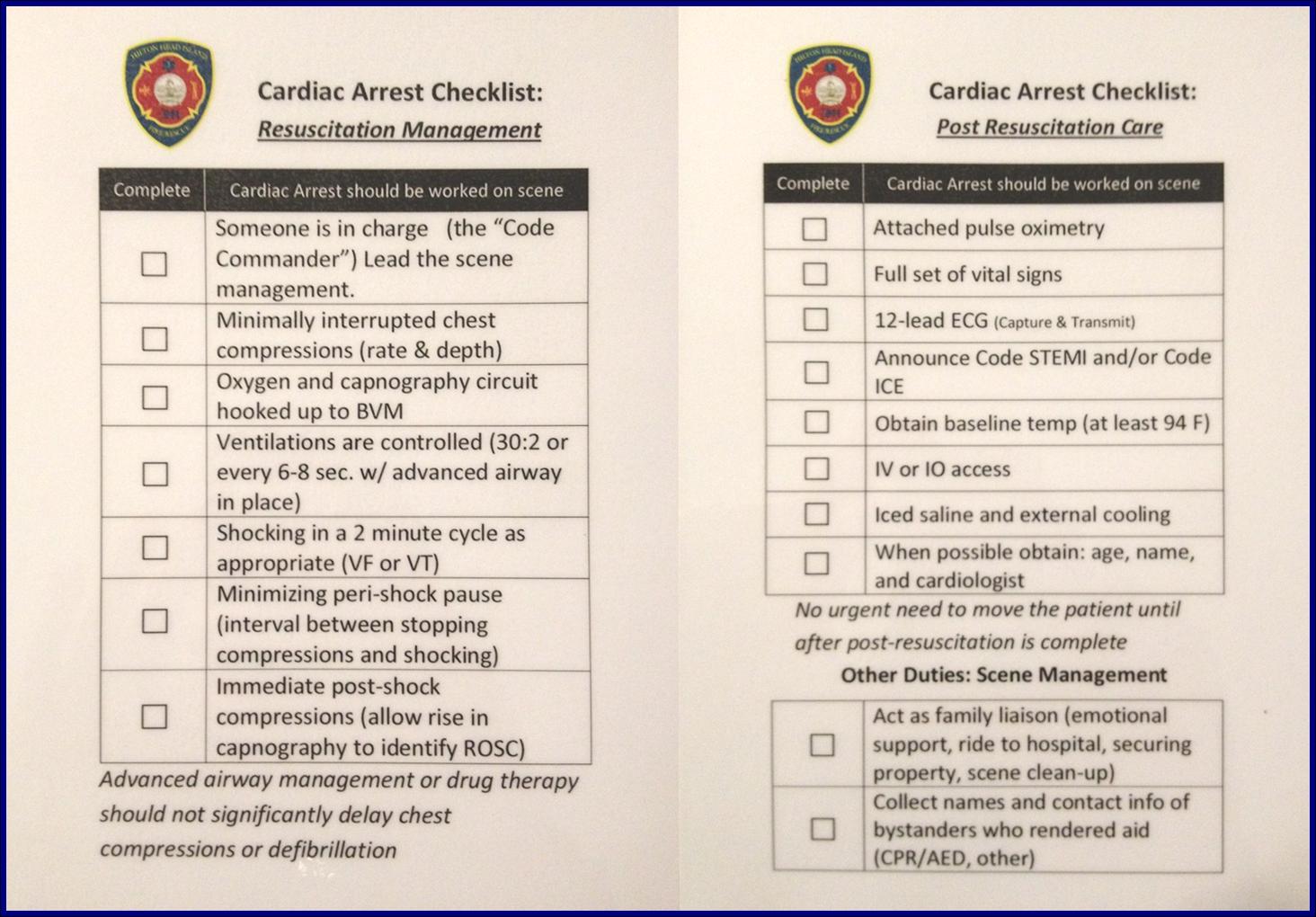

Cardiac Arrest Checklist
D: DYNAMIC PRIORITISATION, DECISION MAKING & DELEGATION
Do you have enough help? Does everyone have a task?
Based on your awareness and anticipation of the resuscitation be decisive on your immediate priorities, make and execute a plan of action, but remember to also be prepared to change as required.
Ensure you have enough resources available for everybody to be able to concentrate on their task fully and not be overloaded. As the resuscitation progresses it is likely your priorities will change and require you to adapt to these and redistribute the workload accordingly.
E: ERROR WISDOM & ENVIRONMENT
What are the potential errors that could occur? Can you carry out effective resuscitation within the environment the patient is in? What needs to change?
‘Forewarned is forearmed’
Be aware of the potential for errors. During stressful events such as resuscitation attempts, with a number of interventions performed and drugs administered, it is easy for these to occur. Considering this risk beforehand and utilizing tools such as checklists and pocket books can help minimize the potential for mistakes and refresh things in your head that you may not encounter very often in practice.
If the patient you are attending is in an unusual environment, or a scene that may pose a danger, consider the options you have to minimize hazards and allow you and the team to carry out the resuscitation safely. Even in domestic locations, during the initial stages of resuscitation it is easy to forget about the environment you are working in. Ensure you have enough space to work in and if not, move something!
I hope you have found this interesting. It may all seem like common sense and I am sure we all give these factors consideration every day with every incident we attend, however personally I feel having a structured approach is worth thinking about to reduce mental overload in times when we all need a little bit of extra brain capacity!
Reference
Logarajah, S. and Alinier, G. (2014). An Integrated ABCDE Approach To Managing Medical Emergencies Using CRM Principles (PDF – Subscription Required). Journal of Paramedic Practice, 6(12), pp.620-625.